In this tutorial, we will learn about the concepts of Electric Charge and Electricity. These are two of the basic concepts of physics that are applicable to a wide range of Engineering studies.
Outline
ToggleIntroduction
You might have had the experience of seeing a spark or hearing a crackle when you take off your clothes (particularly woolen clothes in dry weather) or when touching a door knob to open the door.
The reason for these (and several other) experiences is very simple. When we come in contact (usually, rubbing against) with insulating surfaces, we accumulate Electric Charge. When we touch the door knob or woolen sweater, we are discharging this electric charge through our body.
So, what is this Electric Charge? Why is it important to Physicists and Engineers? Let us try to understand the same in this tutorial.
What is Electric Charge?
Charge is a fundamental property of matter. The amount of charge possessed or carried by a particle is a determining factor on how it reacts to electric and magnetic fields.
Why the reaction to Electric and Magnetic Fields is important? Let me try to explain this as simply as possible. Basically, there are four types of fundamental forces in nature. They are:
- Weak Force
- Strong Force
- Gravity
- Electromagnetic Force
First, lets put gravity aside. Gravitation is a force exerted by two objects, that are astronomical in size, on each other. Gravity is negligible for interactions between two small things at small distances.
The first two i.e. weak and string forces are sub-atomic in nature i.e. they are nuclear in nature. They are usually associated with radioactivity.
So, the only force that we experience (apart from the Earth’s gravity pull) is Electromagnetic Force and it is associated with the fundamental property of matter: The Charge.
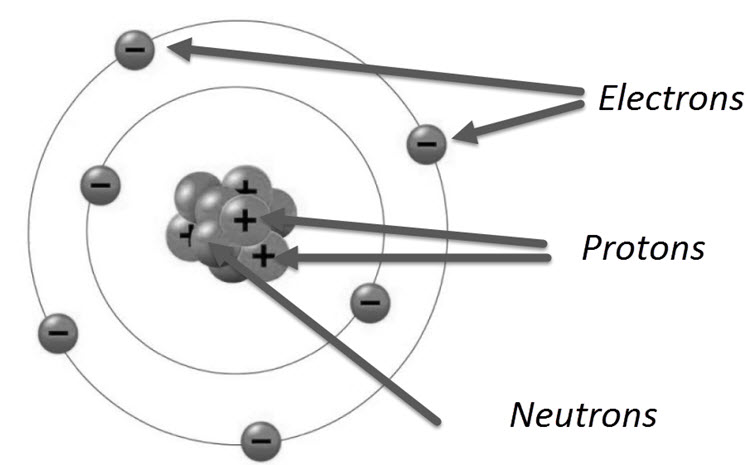
Structure of an Atom
All atoms in nature (apart from Hydrogen) are made up of three basic particles: the electron, the proton and the neutron. The mass of electron and proton are 9.11 x 10-31 kg and 1.6 x 10-27 kg respectively.
Electrons have a negative electric charge and protons have a positive electric charge. Neutrons on the other hand, have a mass similar to that of a proton but has not electric charge.
The center of an atom is called the Nucleus and consists of Neutrons and Protons. Orbiting this nucleus are the electrons. The number of electrons orbiting the nucleus is equal to the number of protons in the nucleus. Hence, electrically speaking, an atom is neutral.
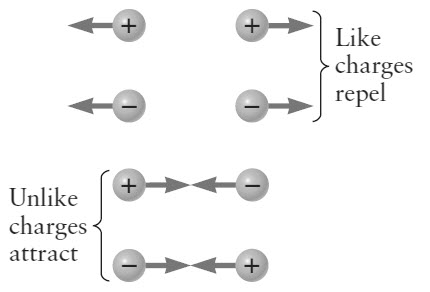
If somehow, some electrons are removed from the atom, then it has lost some negative charge. So, it becomes positively charged body. In contrast, if we somehow ass electrons to an atom, it would have gained extra negative charge and hence it becomes a negatively charged body. Positively charged bodies attract negatively charged bodies and repels positively charged bodies.
Finally, the charge of a body is the count of total surplus or deficiency of electrons in the body. The symbol of Electric Charge is ‘Q’ or ‘q’ and in the honour of physicist Charles de Coulomb, the SI unit of Electric Charge is Coulomb (C).
The smallest amount of “free” charge available in nature is the charge of electron or proton with a magnitude of 1.6 x 10-19 C. Electrons have one unit of negative charge while protons have one unit of positive charge.
To make up 1 C of charge, 1/1.6 x 10-19 or 6.25 x 1018 electrons are required.
Basic Properties of Electric Charge
So, from the above discussion, we have established that there are basically two types of charges: positive and negative. Let us see some of the important properties of Electric Charges.
Assumption: An assumption for the following discussion is that all the charge of the body is concentrated at one point in space. Such charged bodies, where their sizes are smaller that the distance between them, are called Point charges.
Additivity of Charges
If a system contains two point charges then the total charge of the system can be obtained by simply adding the individual charges. Charge has magnitude but no direction i.e. it can either positive or negative.
So, while adding, the proper sign i.e. + or – has to be included in the equation.
Law of Conservation
Earlier we have seen that when we rub a body, it acquires a charge i.e. there is a transfer of electrons from one body to other. No new charges are created or the existing ones are destroyed. This is the Law of Conservation of Charge.
According to this law, you cannot create or destroy charge but only transfer charge from one body to other.
Quantization of Charge
All the electric charge we find in nature is an integral multiple of the magnitude of charge carried by an electron. The charge of electron, which is the basic unit of charge, is denoted by ‘e’.
So, the charge of a body is given by q = ne.
Quantization of charge is the fact that electric charge is always an integral multiple of ‘e’.
Conductors and Insulator
I have spoken that the electrons orbiting the nucleus can either be added or removed to make it negatively or positively charged body. But the ease at which the electrons move is dependent on the type of material.
Basically, all materials in nature are Conductors or Insulators (there is third category called semiconductors, but we will not go into that discussion). Conductors are materials where the electrons (the ones that are farther from the nucleus) can freely move through the body.
Insulators on the other hand, do not allow the movement of electrons as they are held tightly.
Electricity or Electric Current
When Electric Charges are in motion, then they create an electric current, which we simply call as Electricity. Let us go back to door knob situation. Assume, you are rubbing your feet on a carpet. This action will transfer electrons to your body and is building a charge on your body.
Now, if you touch a conductor like door knob in this case, the charge accumulated on your body is transferred to the knob. As the electrons leave your body, you feel a shock.
The reason for this transfer is because the nature wants to balance the situation i.e. a neutral charge equilibrium.
Now coming back to electricity, an electric current is nothing but a charge in motion. The term current is a measure of how much charge is flowing in a unit time.
The symbol of Electric Current is I and is measured in Ampere. One Ampere of current is achieved when one Coulomb of charge is passing through a conductor in one second.
I = Q / t
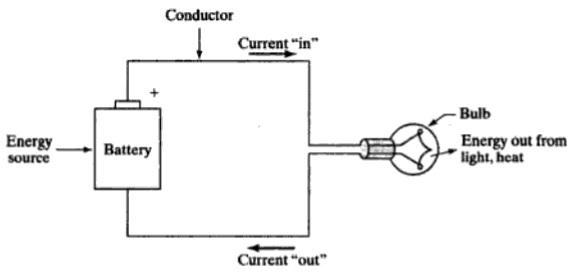
If we consider a conductor like Copper for example, the electrons can freely move between the copper atoms in any direction. Using this property, you can make a closed electric circuit consisting of a copper wire, a light bulb and a battery.
The voltage of the battery will make the electrons to flow in a particular direction and the light bulb glows.