Batteries are more important than ever. As we are searching for ways to reduce carbon emissions, the combination of renewable energy and batteries is the epicenter of all this. Batteries are not something new. We have been using them for a long time. But the technological advancement in the last couple of decades has been tremendous. While electric cars and solar power systems are generally the “larger” applications of batteries, we have the small and portable set of applications as well. We are not talking about fancy smartphones or tablets with Lithium-ion batteries but a much more “millennial”. To be specific, we are talking about AA and AAA Batteries.
Even today, we find ourselves constantly replacing (and sometimes recharging) these tiny batteries from our wireless mice or keyboards, wall clocks, remote controls, flashlights, test and measurement equipment, etc.
As these batteries play an important part in our lives, we decided to make a simple guide comparing AA vs AAA Batteries. Here, we will understand the basics of both AA and AA Batteries, find out their differences, and many more things.
Outline
ToggleA Brief Note on Batteries
Before jumping into the AA vs AAA batteries comparison, let us take things slow and have a brief understanding of battery.
What is a Battery? A Battery is an electrochemical device that converts chemical energy to electrical energy. Technically, a battery consists of one or more cells, the basic electrochemical unit that is made up of electrodes (anode and cathode) and an electrolyte.
The basic classification of batteries consists of only two types; Primary and Secondary. In a Primary Battery, the electrochemical process is irreversible i.e., once we utilize all its chemical energy, there is no way to reverse it.
So, we have to discard these batteries and replace them with new batteries. In contrast to this, in a Secondary Battery, we can reverse the electrochemical process by “recharging” them.
While using Secondary Batteries, we use their chemical energy to provide electrical energy to a load. While recharging, we apply electrical energy to rearrange the chemistry of the cells so that we can use them once again.
Hence, we call Primary Batteries Non-Rechargeable Batteries or Disposable Batteries and Secondary Batteries as Rechargeable Batteries.
There are several chemistries, electrode types, electrolytes, shapes, and sizes in both Primary and Secondary Batteries.
What is an AA Battery?
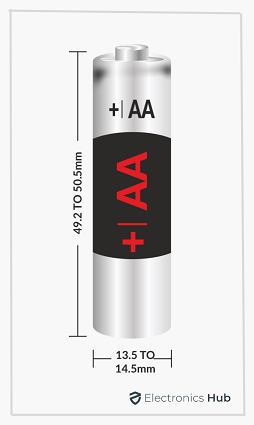
The term “AA” is only an indication of the size of the Battery i.e., its physical dimensions. The shape of an AA Battery is a small cylinder and hence when we talk about its dimensions, we usually mean its height and diameter.
American National Standards Institute or ANSI formally standardized the size of AA Battery as follows:
- The height or length of the AA Tube is 49.2 mm minimum and 50.5 mm maximum
- The diameter of the AA Tube is 13.5 mm minimum and 14.5 mm maximum
Apart from these two, there are two other important dimensions associated with AA Batteries. They are the height and diameter of the positive terminal and the diameter of the negative terminal.
The height of the positive terminal must be at least 1 mm and its diameter can be up to 5.5 mm. Coming to the flat negative terminal, its diameter must be at least 7 mm.
AA Batteries have only one cell in them but they can have different anode, cathode or electrolyte chemistries and compositions. Irrespective of the chemistry of the cell, all AA Batteries are “Dry Cells” as their electrolyte is not liquid (usually a semi-solid gel or paste).
Types of AA Batteries
You can get AA Batteries as both Primary and Secondary types. The potential difference between the electrodes in a typical Primary AA Battery, which we also call the Battery Voltage, is 1.5V and in the case of a Secondary AA Battery, it is 1.2V.
Zinc-Carbon is a very basic and popular type of primary AA Battery. In this, Zinc is the anode and a combination of Carbon (graphite rod) and manganese dioxide acts as the cathode.
Ammonium Chloride is a popular choice for electrolyte in these batteries while some batteries marketed as “heavy duty” have Zinc Chloride as an electrolyte.
Both these electrolytes are acidic in nature. In contrast to these, some Primary AA Batteries use Potassium Hydroxide, which is alkaline, as an electrolyte. These batteries are popularly known as Alkaline Batteries.
The electrodes in Alkaline Batteries are usually the same as the regular AA batteries.
When it comes to secondary or rechargeable AA Batteries, we have two popular options in the form of Nickel Cadmium (NiCd) and Nickel Metal Hydride (NiMH) batteries.
In NiCd batteries, the Cadmium metal acts as the anode while Nickel Oxide Hydroxide acts as the cathode. The electrolyte is usually Potassium Hydroxide (alkaline).
Nowadays, NiCd batteries are being replaced by much better Nickel Metal Hydride batteries. In this type, the cathode is the same Nickel Oxide Hydroxide.
But the anode is a metallic alloy where one metal is one of neodymium, cerium, or lanthanum and the other metal is one of nickel, aluminum, cobalt, or manganese. Even here, the electrolyte is Potassium Hydroxide.
Apart from the two types of primary AA and two types of secondary AA batteries, we also have some not-so-popular types such as Lithium Iron Disulfide – Li-FeS2 (primary), Lithium-ion – Li-ion (secondary), and Nickel Zinc – NiZn (secondary) batteries.
The following table shows some essential information about the four popular types of AA Batteries.
| Category | Battery Type | Anode | Cathode | Electrolyte | Typical Capacity | Voltage |
| Primary (Non-rechargeable) | Zinc Carbon | Zinc | Manganese Dioxide | Ammonium Chloride (or Zinc Chloride) | 400 to 700 mAh | 1.5V |
| Alkaline | Zinc | Manganese Dioxide | Potassium Hydroxide | 1,500 to 3,000 mAh | 1.5V | |
| Secondary (Rechargeable) | Nickel Cadmium | Cadmium | Nickel Oxide Hydroxide | Potassium Hydroxide | 600 to 1,000 mAh | 1.2V |
| Nickel Metal Hydride | Metal Alloy | Nickel Oxide Hydroxide | Potassium Hydroxide | 6,00 to 3,000 mAh | 1.2V |
What is AAA Battery?
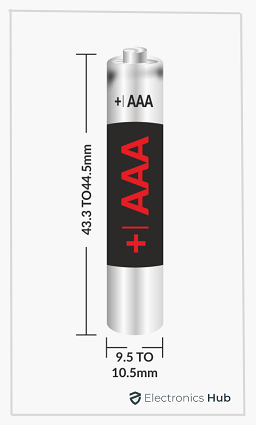
The AAA Battery is also cylindrical. But the height (or length) and the diameter of the AAA Battery are less than the AA Battery. As per ANSI standards, the following are the dimensions of AAA Batteries.
- The height or length of the AAA Tube is 43.3 mm minimum and 44.5 mm maximum
- The diameter of the AAA Tube is 9.5 mm minimum and 10.5 mm maximum
The minimum height of the positive terminal must be 0.8 mm while its maximum diameter can be 3.8 mm. The minimum diameter of the flat negative terminal is 4.3 mm.
Similar to AA Batteries, even AAA Batteries are available in both primary (non-rechargeable) and secondary (rechargeable) types.
The four types we discussed in the AA Battery category are also available in AAA Sizes. Electrodes, electrolytes, and voltages of AAA Batteries are the same as AA Batteries. The only difference is the size.
AAA Batteries are slightly smaller than AA Batteries. Due to the reduction in the physical size of the battery, the size of electrodes and the quantity of electrolytes becomes less. As a result, the capacity of AAA batteries is lower than its AA counterparts.
The following table shows the typical capacities of the common and popular AAA Batteries.
| Category | Battery Type | Typical Capacity |
| Primary (Non-rechargeable) | Zinc Carbon | 500 to 600 mAh |
| Alkaline | 800 to 1,300 mAh | |
| Secondary (Rechargeable) | Nickel Cadmium | 300 to 500 mAh |
| Nickel Metal Hydride | 600 to 1,300 mAh |
Differences: AA vs AAA Batteries
From the above discussion, it is clear that the main difference between AA and AAA Batteries is their physical size. But in order to make a decent AA vs AAA Batteries comparison, we have to explore some other differences (or similarities) between them.
1. Size
The first and obvious difference between AA and AAA batteries is their size. AA Batteries are slightly longer and wider than their AAA counterparts. If you compare the height or lengths of AA and AAA Batteries, it is approximately 50.5 mm (+0/-1.3mm) for AA and 44.5 mm (+0/-1mm) for AAA.
Coming to their diameters, AA batteries have 14.5 mm (+0/-1mm) while AAA batteries have 10.5 mm (+0/-1mm).
2. Chemistry
The overall chemistry of the cell in AA and AAA batteries is identical. Both have similar types of anodes, cathodes, and electrolytes.
As the size of AAA batteries is smaller than AA batteries, the amount of chemicals is also less. This has a significant impact on the chemical energy that these batteries can hold.
The chemistry of the batteries has also an impact on their shelf life. If you take Zinc Carbon type batteries, both AA and AAA types have a shelf life of anywhere between 3 to 5 years.
But if you take Alkaline Batteries, both AA and AAA types have a much longer shelf life of 5 to 10 years.
3. Capacity and Runtime
Continuing on the size and chemistry, the slightly larger size of AA battery means, it contains more energy than their AAA counterparts.
As an example, if you take the popular Alkaline batteries, AA batteries can have a maximum capacity of 3,000 mAh while it is only 1,300 mAh AAA batteries.
As a result, if you compare AA and AAA batteries with similar power draws, then AA batteries will last almost twice longer as AAA batteries.
4. Applications
AA batteries, due to their higher energy capacity, are commonly used in slightly power-hungry applications such as lighting, motor toys, photography equipment, etc.
On the other hand, AAA batteries, due to their lower energy capacity, are often used in remote controls, game controllers, etc.
Nowadays, lithium-ion (or its types) are replacing AA and AAA batteries in flashlights, cameras, etc. due to their high energy density. But AA and AAA batteries are still popular in wireless mice and keyboards, remote controls, wall clocks, smoke sensors, multimeters, and other similar applications.
5. Cost
If you make an apples-to-apples comparison i.e., AA and AAA batteries of the same type (both Zinc Carbon or both Alkaline), then AA batteries are slightly pricy than AAA batteries.
But if you compare with AA (or AAA) i.e., AA Zinc Carbon vs AA Alkaline (or AAA Zinc Carbon vs AAA Alkaline), then Alkaline batteries are expensive types.
Comparison of AA and AAA Batteries
| Battery | Chemical Composition | AA | AAA | ||||||
| IEC Nomenclature | ANSI Nomenclature | Dimensions | Typical Capacity | IEC Nomenclature | ANSI Nomenclature | Dimensions | Typical Capacity | ||
| Primary (Non-rechargeable) | Zinc Carbon | R6 | 15D | Height (Length) – 49.2 mm (min) and 50.5 mm (max)
Diameter – 13.5 mm (min) and 14.5 mm (max) |
400 to 700 mAh | R03 | 24D | Height (Length) – 43.3 mm (min) and 44.5 mm (max)
Diameter – 9.5 mm (min) and 10.5 mm (max) |
500 to 600 mAh |
| Alkaline | LR6 | 15A | 1,500 to 3,000 mAh | LR03 | 24A | 800 to 1,300 mAh | |||
| Secondary (Rechargeable) | Nickel Cadmium | KR6 | 15K | 600 to 1,000 mAh | KR03 | 24K | 300 to 500 mAh | ||
| Nickel Metal Hydride | HR6 | 15H | 6,00 to 3,000 mAh | HR03 | 24H | 600 to 1,300 mAh | |||
Can We Use AAA Instead of AAA or Vice-Versa?
This is a very common question. But sadly, the answer is no. Despite having the same chemistry, AA and AAA batteries are physically incompatible with each other.
For instance, you have a wall clock that has a holder for AA-sized batteries. Then you cannot put a AAA battery in this holder as they are slightly smaller.
Similarly, you cannot put AA batteries in AAA holders as well.
Sometimes, compatibility within AA and AAA batteries is also absent. We are talking about non-rechargeable and rechargeable batteries.
We know that non-rechargeable batteries of both AA and AAA type have a voltage of 1.5V. But the voltage of the rechargeable type (of both AA and AAA) is only 1.2V.
So, depending on the application, this voltage difference might or might not be a problem. A popular scenario is remote controls for modern smart TVs. Most Smart TV manufacturers recommend AA Alkaline batteries for their “Smart” remote controllers, usually two (which are in series).
The two AA Alkaline batteries in series have a voltage of 3V. But if you take two rechargeable AA batteries in series, the voltage is only 2.4V. The remote control might see this as a low voltage and doesn’t turn on or might have a very short runtime.
Hence, you have to check twice before you replace batteries and make sure that the replacement ones are compatible with the application.
Conclusion
Batteries are an essential part of our lives. We use them everywhere be it clocks, flashlights, remote controls, toys, etc. While the popularity of lithium-ion batteries is ever-increasing, regular AA and AAA batteries are still popular for several applications.
In this guide, we saw the basics of both AA and AAA batteries. We looked at different types of AA and AAA batteries, their important parameters, and their specifications.
After that, we made a simple AA vs AAA Batteries comparison by finding the differences and similarities between the two. We hope that this guide on AA vs AAA batteries could help you understand more about these common household batteries.
If you feel we missed something or want us to add anything, do let us know in the comments section. It will not only help us but even other readers.

